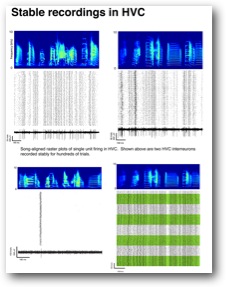
Figure. Single unit recordings of a sparse firing projection neuron and three interneurons. Green bands in lower right reveal boundaries of separate days. Firing patterns are stable.
Chronically implanted microelectrodes arrays are an essential tool in fundamental neuroscience research. These devices also may one day provide routine intracranial brain-machine interfaces in humans, restoring movement and communication to people whose interaction with the physical world is either completely eliminated due to stroke or disease or impaired due to spinal cord injury or amputation. Proof of principle experiments have demonstrated control of robotic limbs through intracranial electrodes, and efforts around the world are building toward a goal of monitoring brain function with vast numbers of probes that sense neural activity with high precision, revealing principles of the human mind. However, monitoring large ensembles of neural activity in the living brain with single neuron and single spike resolution faces challenging technical limitations. For microelectrodes, the biggest limitation of chronic neural recording is a reactive tissue response that encapsulates electrodes and kills or damages neurons. This rejection of electrode implants leads to particularly serious problems when recording from densely packed neurons in small animal research models, or when recording over the multi-year time-scales required for practical human neural prosthetics. This project develops a novel multielectrode array that is designed to be minimally invasive, while still providing stable recordings from many neurons simultaneously.
The proposed technology is a “tunneling fiber array” consisting of dense bundles of ultra-small sharpened carbon fibers that can be discretely inserted into the brain. The key feature of the design is observed during implantation of the electrode; rather than tearing through tissue in the un-compliant manner of existing commercial arrays, the proposed array splays during insertion and individual threads are free to follow their own path of least resistance into the brain. Neural recordings from prototype devices are stable over a time-scale of months. This project aims to directly test how electrode splaying contributes to chronic recording stability, and to examine the interface between brain tissue and electrode through in-vivo imaging and histology. The central hypothesis is that the tunneling fiber array shows reduced tissue damage due to the small scale fibers and due to the ability of single electrodes to separate from each other during implant. Over long time-scales these features are hypothesized to minimize damage to neurons and blood vessels in the space adjacent to the fibers, promoting stable recordings. This project holds the potential to enable new fundamental research that requires long term recordings with single cell resolution. The proposed work does not address issues specifically related to human neural prosthetics, but since the small animal model is a particularly challenging test bed for chronic recording, the results of this study are likely to inform future designs for minimally invasive electrodes more generally.
Aim 1. Quantify the impact of fiber splaying on the yield and stability of neural recordings. Preliminary data has shown excellent signal longevity, and the splaying of fibers may allow implantation of high channel count arrays while minimizing tissue damage. The project will quantify the yield and stability of multi-unit and single unit recordings as a function of the number of contacts and as a function of tip geometry. These numbers will be compared to a monolithic version of the electrode that prohibits individual fibers from splaying.
Aim 2. Test whether the tunneling arrays are deflected around vasculature. The independent “splaying” of the electrode threads is hypothesized to reduce vascular damage relative to a monolithic bundle of fibers that cannot splay. The project will examine the process of electrode insertion through in-vivo two photon imaging, and by reconstruction of electrode paths in deep tissue through subsequent histological sectioning. Information gained will inform the scalability of the design for high channel count arrays.
Aim 3. Test whether tunneling fiber arrays form a minimally invasive interface with the brain in long term chronic implants. This aim examines histological markers of gliosis, neuronal and synaptic health, and the cerebral vasculature for chronic implants at the three month time point. Histological results will be cross referenced with the yield and stability of chronic neural recordings (Aim 1). Control comparisons will include monolithic fiber bundles that cannot splay, and splaying fiber bundles that are free-floating in the brain, untethered to the skull. The result is anticipated to reveal minimal tissue damage over long time-scales and will establish parameters such as fiber count for individual bundles that maximize neuronal yield and stability.