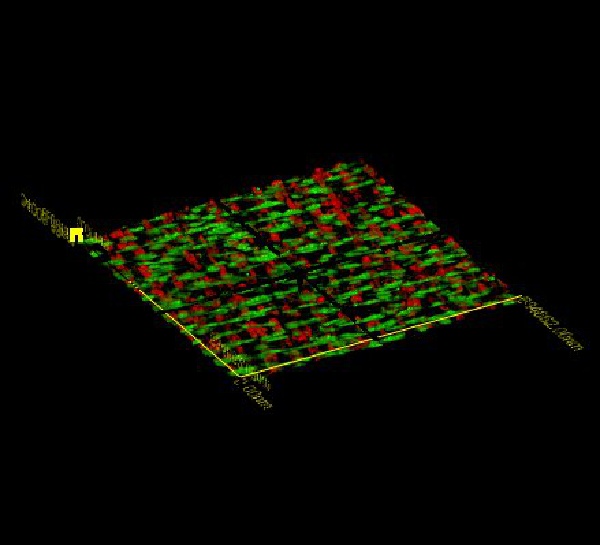
The main goal of this research is to develop tissue engineering solutions for surgical reconstruction of blood vessels in pediatric patients. Currently, materials used for such surgical procedures do not have the capacity to grow with the child and frequently fail, and children therefore often require repeat surgeries as they mature. Grafts using tissue engineering approaches have the potential to grow with the child and therefore would have tremendous impact on clinical practice. The picture above are stacked sheets of cells with their associated extracellular matrix (proteins). The sheets are micropatterned; thus we can manipulate their structure, and ideally their function. We are grateful for funding from The Hartwell Foundation, the National Science Foundation, and the National Institutes of Health.
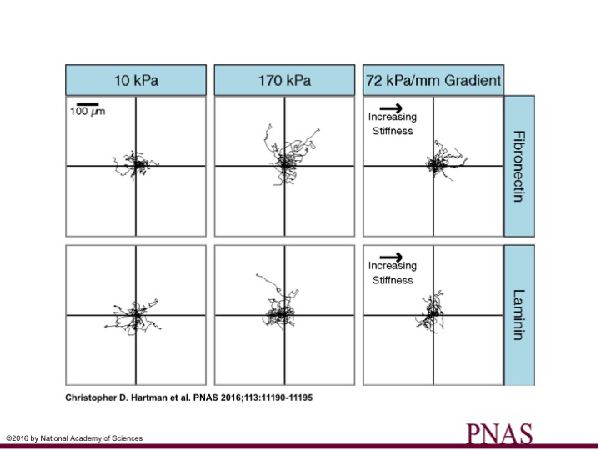
Similarly to how cells migrate in response to gradients in chemical factors (chemotaxis), cells can also migrate in response to gradients in mechanical factors (durotaxis). In previous studies, we have observed that vascular smooth muscle cells (VSMCs) undergo durotaxis. This phenomenon has potential implications in cardiovascular disease, and because we also observed that VSMCs behaved differently depending on which proteins they were attached to, we hypothesized that the durotactic phenomenon would also depend on protein composition. The above figure shows that VSMCs undergo durotaxis on gradient stiffness substrates coated with fibronectin, but not those coated with laminin. We are grateful for funding the National Institutes of Health.
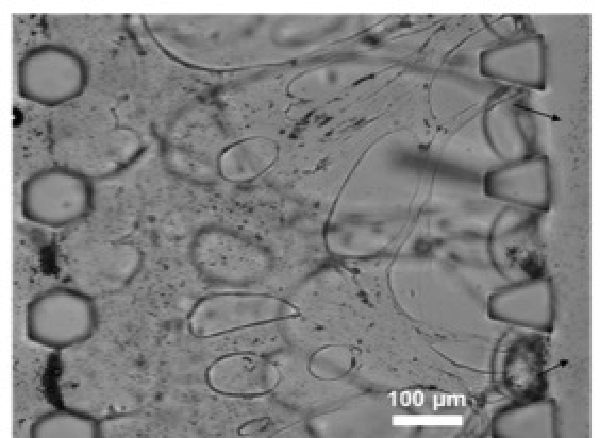
We have developed in collaboration with Nooli Jeon's group at Seoul National University (South Korea) a platform to test ultrasound contrast agents together with drug delivery vehicles. Such platforms are attractive because it allows pre-screening and testing and optimization of contrast agents and drug delivery platforms on a chip. These studies can be done before animal studies are done, and allow us to test various parameters that can be difficult to tease apart in an animal model. The above image is of endothelial cells that have formed a complex network in a fibrin matrix. We can flow through microbubbles (ultrasound contrast) and liposomes that carry drug payloads. We are grateful for funding from Boston University's College of Engineering.
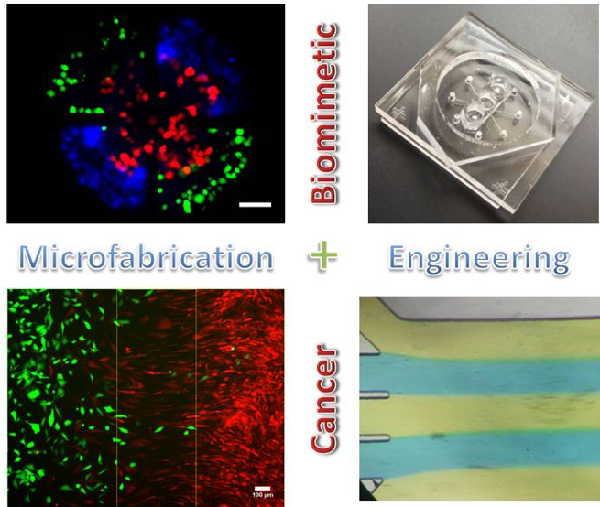
Cancer metastasis is a major cause of cancer deaths, and we would like to understand how certain cancer cells metastasize to specific organ types. Similar to the 'Blood Vessel on a Chip' project, we use microchips to do pre-screening in a simple system that mimics certain elements of cancer cells interacting with their tumor microenvironment. The above image shows different panels illustrating our general approach. We can label different cell types with different color dyes and then place them in close proximity to each other to observe their influences on each other over time using microscopy. We hope ultimately to be able to use patients' cancer cells to predict metastasis in our microchips. We are grateful for funding from the National Science Foundation and the National Institutes of Health.