
Willmen Youngsaye B.S. Biochemistry, Tufts University, 2003 Boston University, Fall 2003

Willmen Youngsaye B.S. Biochemistry, Tufts University, 2003 Boston University, Fall 2003
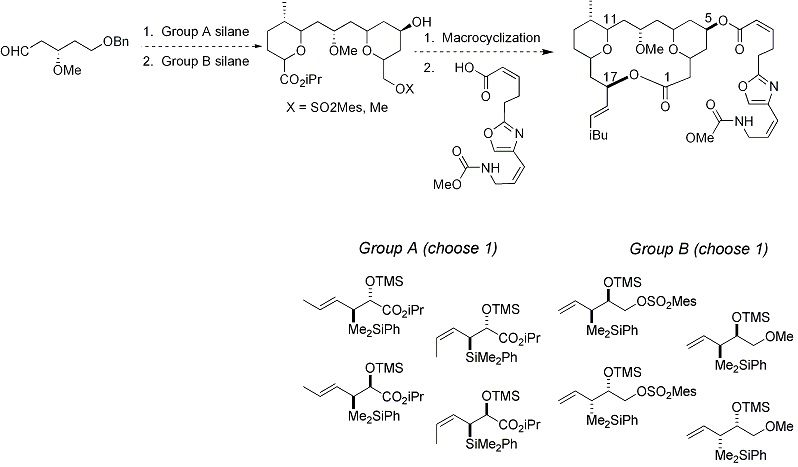
Development of a Leucascandrolide A diastereomeric library
 |
|||
| Key to Library Development Trying different combinations of crotylsilanes A , allylsilanes B , and C-17 epimers can produce up to 32 diastereoisomers of leucascandrolide A. Epimerizing the C-3, C-7, C-11, C-15, & C-17 centers allows us to influence directly the three-dimensional conformation of the macrolactone ring system. This presents the opportunity to probe its structure/activity relationship and perhaps influence its inherent toxicity against the human KB tumor cell line or even induce a new biological effect. |