Ingredients: fine powdered zinc, fine powdered sulfur
Procedure: A minimal recipe follows.
1. Measure out zinc and sulfur in a 1:1 molar ratio. A rough approximation might be 6.5 g of zinc and 3.2 g of sulfur.
2. Mix the zinc and sulfur powders together thoroughly.
3. Ignite the mixture using a "white hot" metal loop.
Understanding:
The zinc and sulfur react violently, producing a substantial quantity of heat, light, and smoke. The principle reaction is between zinc and sulfur
Zn(s) + S(s)
→ ZnS(s)
However, when the reaction is run under aerobic conditions in air, there is a competing reaction
2 Zn(s) + O2(g)
→ 2 ZnO(s)
 The reaction forms an off-white crystalline powder of zinc sulfide. For many years, zinc sulfide has been known to be phosphorescent. It is often used a phosphor, for example, in paints used on watch dials. As a mineral, it is found as "sphalerite."
The reaction forms an off-white crystalline powder of zinc sulfide. For many years, zinc sulfide has been known to be phosphorescent. It is often used a phosphor, for example, in paints used on watch dials. As a mineral, it is found as "sphalerite."
The reaction of an excess of zinc may lead to formation of zinc oxide, commonly known as "zinc white."
Zinc white is a common pigment found in paints. It is also a principal component of ointments designed to prevent sunburn in the summer, windburn in the winter, and diaper rash ... all year around! In nature, zinc oxide is found as the mineral "zincite."
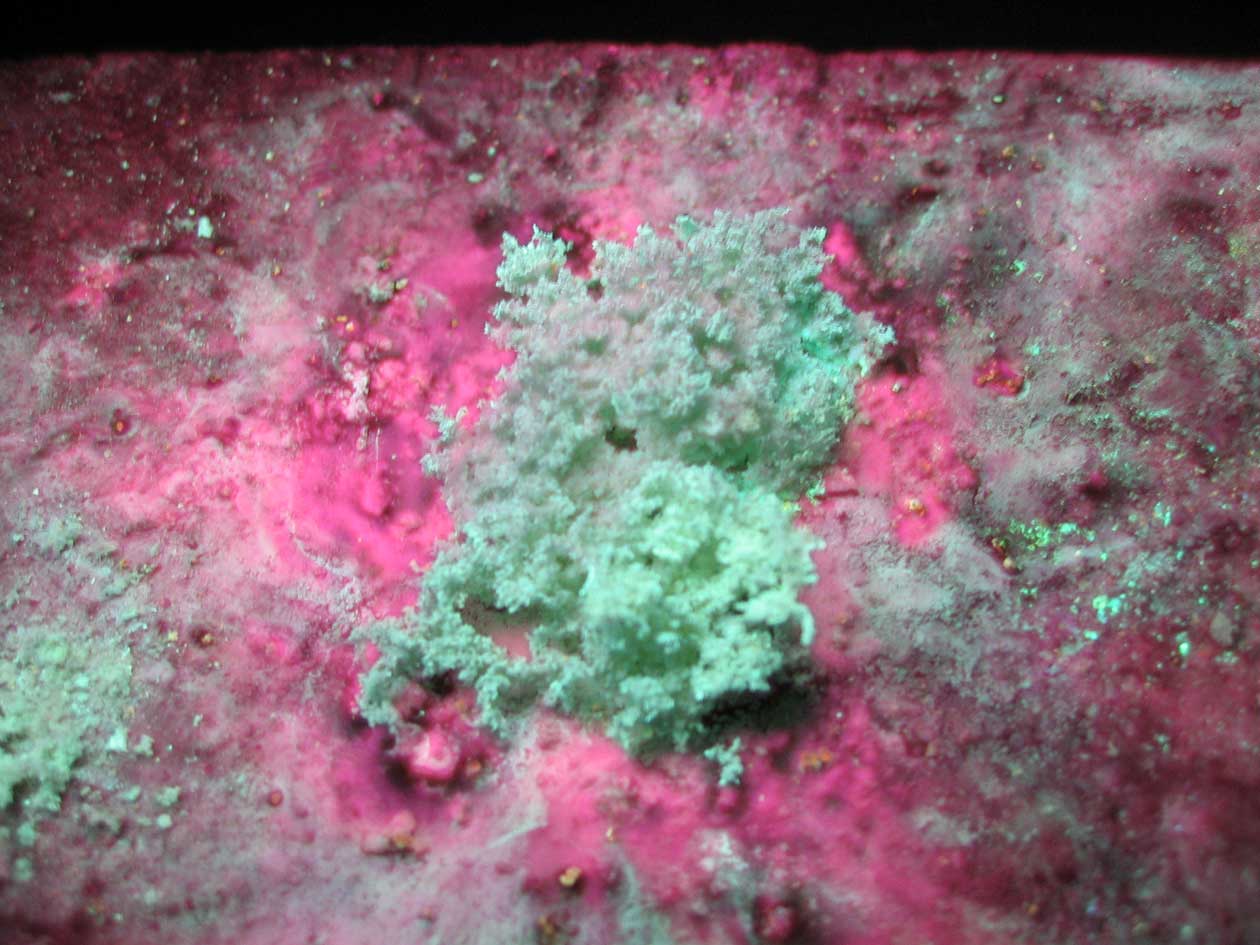 In fluorescence, light energy is absorbed and then rapidly reemitted. In phosphorescence, the light energy is absorbed but then reemitted over a longer period of time. It is the "delay" in the emission of phosphorescence that distinguishes it from fluorescence.
In fluorescence, light energy is absorbed and then rapidly reemitted. In phosphorescence, the light energy is absorbed but then reemitted over a longer period of time. It is the "delay" in the emission of phosphorescence that distinguishes it from fluorescence.
During exposure to short wavelength (254 nm) UV light, the product of our reaction is observed to fluoresce.
The phosphorescent "green" that is seen in the image is the zinc sulfide product.

During exposure to long wavelength (366 nm) UV light, the zinc sulfide is found to fluoresce and phosphoresce with impressive intensity and vibrant color.
After turning the UV light "off" we see the evidence of phosphorescence emission from the zinc sulfide in the form of an "eerie green" glow. The glow is maintain for several seconds.
At the end of the nineteenth century, William Crookes discovered cathode rays. He placed a cathode and anode within a vacuum tube, along with a screen coated with zinc sulfide. Crookes observed a glowing image on the zinc sulfide screen consistent with the hypothesis that "rays" had been emitted by the cathode, causing the zinc sulfide to fluoresce. The ghostly glow that we observed must be much like that observed by Crookes in his discovery of cathode rays more than a century ago.
The fruit of physics
Question: It is difficult to
appreciate the sizes of atoms and molecules using our intuition
based on the world we can see with our naked eyes. To help in this
project, it is useful to find an object whose size relative to
that of the Earth is the same as a the size of a molecule
relative to that object. That is, we want to fill in the ``X'' in
the statement ``an atom is to an X as an X is to the Earth.''
Find such an object!
Useful information: The circumference of the Earth at the equator is
approximately 25,000 miles. 1 mile = 1609 m. A molecule of water
has a diameter of approximately 3.1 Ångströms (1 Ångström = 1 Å = 1 X
10-10m).
You can check your answers here.
 In fluorescence, light energy is absorbed and then rapidly reemitted. In phosphorescence, the light energy is absorbed but then reemitted over a longer period of time. It is the "delay" in the emission of phosphorescence that distinguishes it from fluorescence.
In fluorescence, light energy is absorbed and then rapidly reemitted. In phosphorescence, the light energy is absorbed but then reemitted over a longer period of time. It is the "delay" in the emission of phosphorescence that distinguishes it from fluorescence.
 A mixture of zinc and sulfur reacts spontaneously, under a flash of fire and smoke. The gray-green mixture is transformed into an off-white powder with magical properties.
A mixture of zinc and sulfur reacts spontaneously, under a flash of fire and smoke. The gray-green mixture is transformed into an off-white powder with magical properties.
 The reaction forms an off-white crystalline powder of zinc sulfide. For many years, zinc sulfide has been known to be phosphorescent. It is often used a phosphor, for example, in paints used on watch dials. As a mineral, it is found as "sphalerite."
The reaction forms an off-white crystalline powder of zinc sulfide. For many years, zinc sulfide has been known to be phosphorescent. It is often used a phosphor, for example, in paints used on watch dials. As a mineral, it is found as "sphalerite."
