The titration of a WEAK base with a STRONG acid
The pH of an initial solution of weak base is observed to display a richly structured transition as strong acid is "titrated" to neutralize and then acidify the solution.



Ingredients: sodium hydroxide, ammonia
Procedure: A complete recipe follows.
1. Prepare a 0.0100M aqueous solution of ammonia.
2. Prepare a 0.100M aqueous solution of hydrochloride acid.
3. Titrate 200.0mL of ammonia solution with the hydrochloric acid solution.
4. Observe the change in pH.
Understanding: The chemistry of the titration of the weak base, ammonia, with the strong acid, hydrogen chloride, is captured by the neutralization reaction
NH3(aq) + HCl(aq) → NH4Cl(aq) + H2O(l)
As the essence of the reaction isbase + acid → salt + water
The net ionic equation for the neutralization reaction of the weak base titrated by the strong acid can be writtenNH3(aq) + H+(aq) → NH4+(aq)
In this case, the ammonia is the Lewis base which donates its lone pair of electrons to the proton, which acts as the Lewis acid. The result is the creation of the ammonium ion that is the conjugate acid to the ammonia base.
The regime of "pure" weak base
At the start of the titration, the solution is observed to be basic, with a pH exceeding 9. The dominant species in the solution are the ammonia and water. The equilibrium that determines the pH of the solution is
NH3(aq) + H2O(l) → NH4+(aq) + OH-(aq)
The regime of the "half-equivalence point" and BUFFER solutions
That initial drop in pH is followed by a region of relatively little change in the pH near the half-equivalence point - the point at which the equivalents of acid added to the solution equals one-half of the equivalents of weak base initially present in the solution.
At the half-equivalence point, the concentration of weak base is equal to the concentration of its conjugate acid. In the region of the half-equivalence point, the concentrations of weak base and its conjugate acid are similar.
The dominant species in solution are ammonia, ammonium ion, and water. The equilibrium that determines the pH of the solution is
NH3(aq) + H2O(l) → NH4+(aq) + OH-(aq)
When the strong acid is added to the solution of the weak base and its conjugate weak acid, the strong acid is immediately converted to weak acid through the neutralization reaction
NH3(aq) + H3O+(aq) → NH4+(aq) + H2O(l)
NH4+(aq) + OH-(aq)
→
NH3(aq) + H2O(l)
NH3(aq) + H2O(l) → NH4+(aq) + OH-(aq)
NH3(aq) + H2O(l) ← NH4+(aq) + OH-(aq)
Note that the calculation of the pH of a buffer solution is identical to the calculation of the pH of a solution of the pure weak base, with the sole difference being that in the case of the buffer solution, there is an initial concentration of weak base and its conjugate acid. The mechanics of the calculation, however, are identical!
The regime of the "equivalence point" and HYDROLYSIS
As the titration continues beyond the half-equivalence point, the remaining weak base is depleted and the capacity of the buffer solution is depleted. Approaching the equivalence point, there is a strong drop in the pH of the solution. There is little or no remaining weak base to absorb the added strong acid, and the buffering capacity of the solution has been exceeded.
At the equivalence point, the number of equivalents of strong acid added during the titration is exactly equal to the number of equivalents of weak base initially in the solution. The weak base has been fully converted to its conjugate weak acid by the titration. At the equivalence point, the dominant species in the solution are the ammonium ion and water. The equilibrium that determines the pH of the solution is the hydrolysis of the ammonium ion
NH4+(aq) + H2O(l)
→
NH3(aq) + H3O+(aq)
The regime of excess strong acid
As the titration continues, additional strong acid is added to the solution beyond the equivalence point. The pH of the solution plummets as the buret becomes a faucet of hydrogen ions that are no longer absorbed by the weak base. The dominant species in the solution is the hydrogen ion. The equilibrium that determines the pH is that of the excess strong acid
HCl(aq) + H2O(l)
→
H3O+(aq) + Cl-(aq)
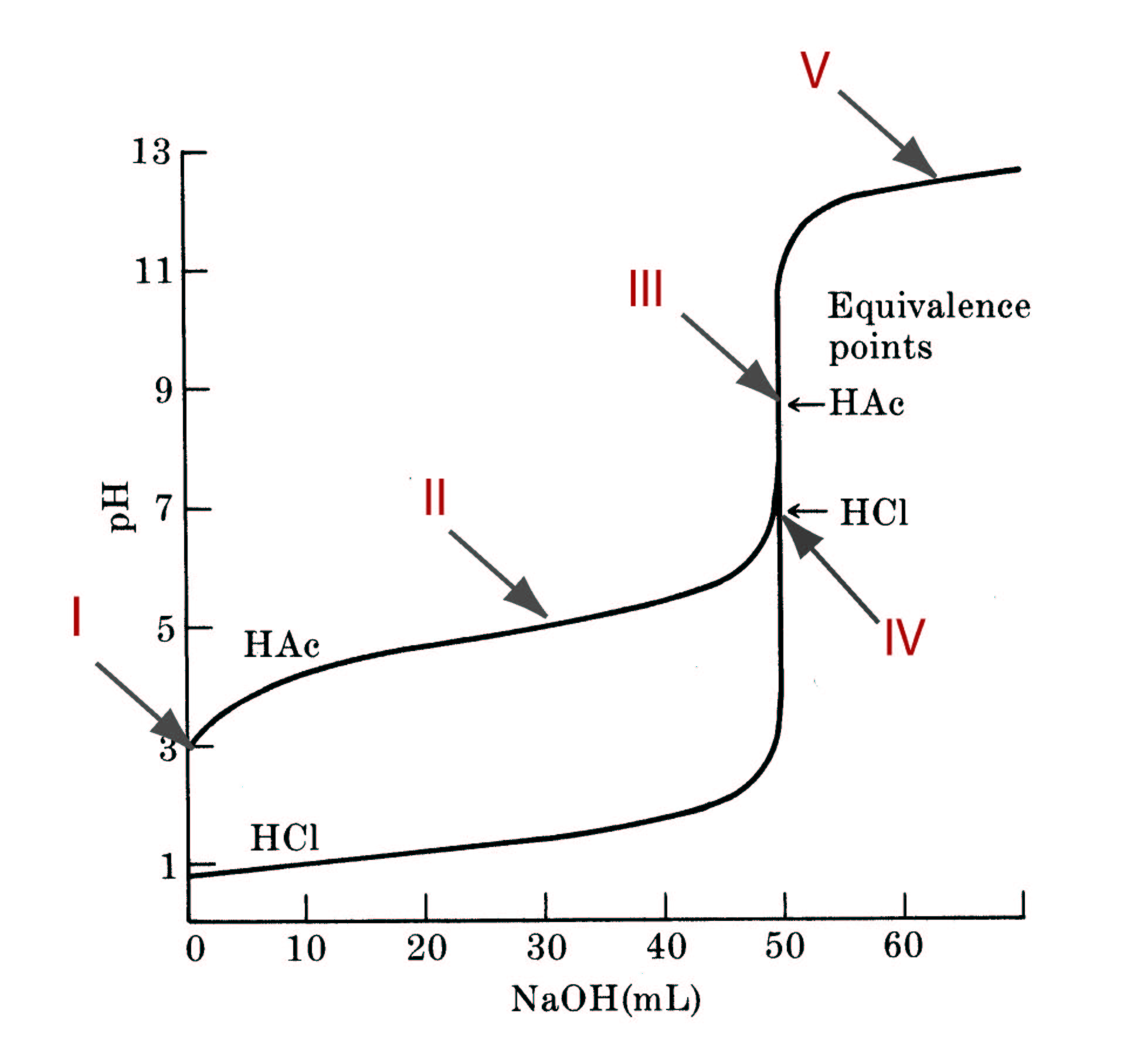
Consider the titration of 50.00mL of a 0.2000M solution of acetic acid with a 0.2000 M solution of sodium hydroxide. The Ka = 1.8 x 10-5 for acetic acid at 25C.
For region I, region II, region III and region V, define (1) the dominant species in the solution, (2) the chemical equilibrium (or excess species) that defines the pH in that region, and (3) the equilibrium constant for that reaction (if appropriate).
Compute the pH of the solution at the following volumes of strong base added in the titration: 0.00, 10.00, 25.00, 40.00, 50.00, 50.01 and 55.00 mL.
You can check your answers here.
Exploring the chemistry of solutions of acids and bases
Question:
In the titration of a weak acid, acetic acid, with the strong base, sodium hydroxide, there is a rich display of chemistry that presents every interesting regime of acid/base equilibria - weak acid/base, buffer, hydrolysis, and strong base/acid.