The titration of a STRONG base with a STRONG acid
The pH of an initial solution of strong base is observed to undergo a precipitous transition as the solution is "titrated" with a solution of strong acid.



Ingredients: sodium hydroxide, hydrochloric acid
Procedure: A complete recipe follows.
1. Prepare a 1.00M aqueous solution of sodium hydroxide.
2. Prepare a 6.00M aqueous solution of hydrochloric acid.
3. Titrate 200.0mL of sodium hydroxide solution with the hydrochloric acid solution.
4. Observe the change in pH.
Understanding: The titration of the strong base, sodium hydroxide, with the strong acid, hydrogen chloride, has at its heart the neutralization reaction
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
The essence of the reaction isbase + acid → salt + water
As the sodium hydroxide and hydrogen chloride are both strong electrolytes, the reaction can also be representedNa+(aq) + OH-(aq) + H+(aq) + Cl-(aq) → Na+(aq) + Cl-(aq) + H2O(l)
The net ionic equation for the neutralization reaction of the strong base titrated by the strong acid can be writtenOH-(aq) + H+(aq) → H2O(l) K = 1/Kw
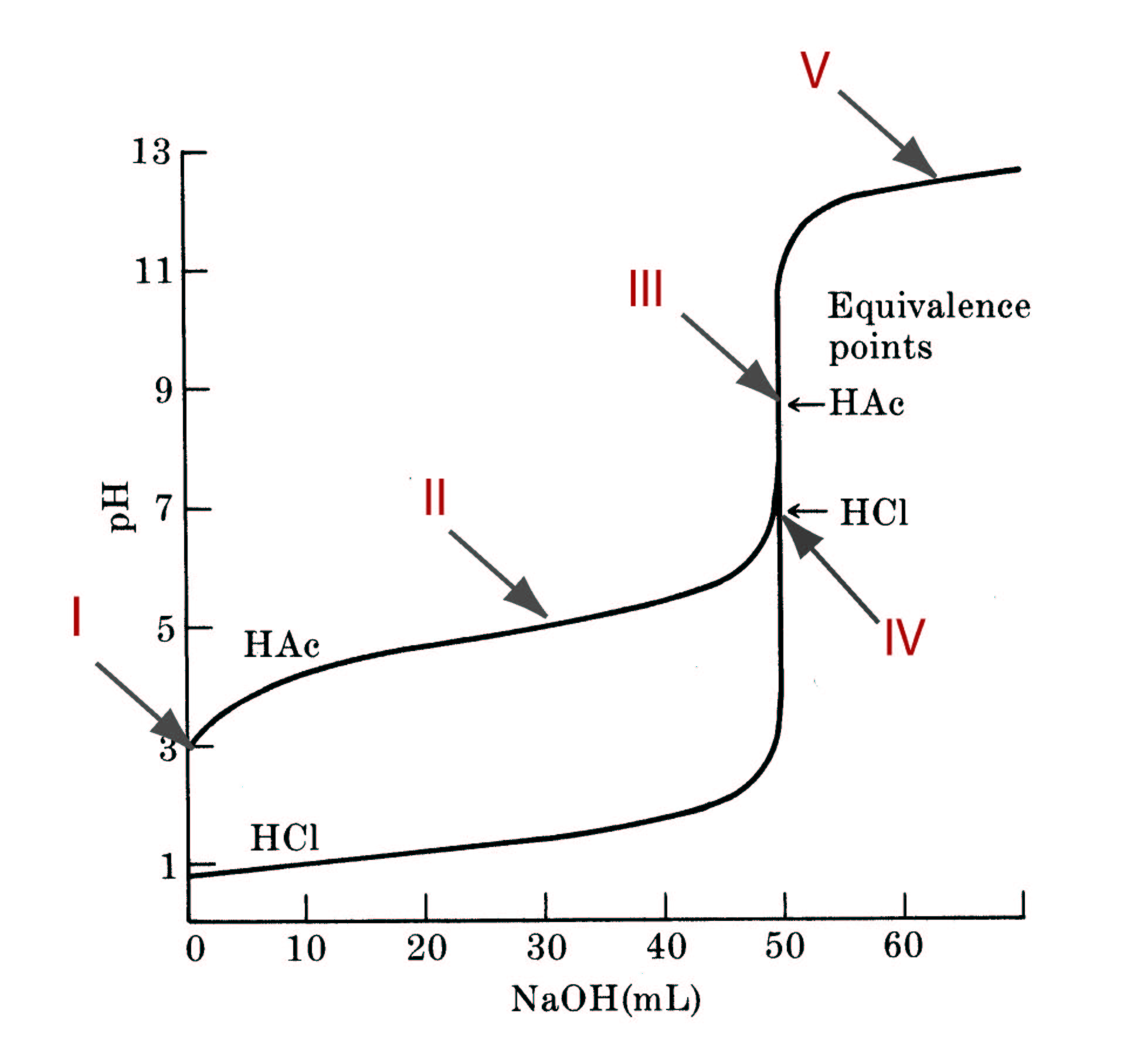
The regime of excess strong baseAt the start of the titration, the solution is observed to be strongly basic, with a pH exceeding 12. As the titration proceeds, the pH of the solution initially diminishes quite slowly. Why slowly? Because any strong acid that is added to the solution is immediately neutralized to salt and water, and the excess strong base keeps the solution strongly basic. The dominant species in the solution is the hydroxide ion, and the solution is basic.
The equivalence point
The initial solution has a certain number of equivalents of strong base. The point at which the number of equivalents of acid added equals the number of equivalents of base initially in the solution is called the equivalence point. As the equivalence point is approached, a dramatic reduction in the pH is observed due to the depletion of excess base. At the equivalence point, there is no excess strong base or strong acid.
The dominant species in the solution is water and the chemical equilibrium that determines the pH of the solution is the autoionization of water
H2O(l)
→
OH-(aq) + H+(aq)
Kw = aH+ aOH- = 1.00 x 10-14 @ 25C
The regime of excess strong acid
As additional strong acid is added to the solution, the formation of excess strong acid causes the pH of the solution to plummet further to the deeply acidic range of pH less than 2. The dominant species in the solution is the excess hydrogen ion, and the pH of the solution is strongly acidic.
Consider the titration of 50.00mL of a 0.2000M solution of hydrochloric acid with a 0.2000 M solution of sodium hydroxide. Take Kw = 1.00 x 10-14.
For the initial strong acid solution, region IV, and region V, define (1) the dominant species in the solution, (2) the chemical equilibrium (or excess species) that defines the pH in that region, and (3) the equilibrium constant expression corresponding to that reaction (if appropriate).
Compute the pH of the solution at the following volumes of strong base added in the titration: 0.00, 10.00, 25.00, 40.00, 49.00, 49.90, 49.99, 50.00, 50.01, and 55.00mL.
You can check your answers here.
QUALitative and QUANTitative understanding of titrations
Question:
In the titration of a strong acid, hydrochloric acid, with the strong base, sodium hydroxide, the chemistry is quite straightforward. The regimes of chemistry are captured by two principal types of equilibria - strong acid/base solutions and the autoionization of water.