Observing dramatic physical transformations mediated by reduction/oxidation reactions
 A metallic copper plate is immersed in a solution of silver nitrate, leading to the formation of a "fur" of metallic silver.
A metallic copper plate is immersed in a solution of silver nitrate, leading to the formation of a "fur" of metallic silver.
Ingredients: silver nitrate, copper bar
Procedure: A complete recipe follows.
1. Prepare a solution of silver nitrate.
2. Place a copper bar in the silver nitrate solution.
3. Allow a few minutes to pass and observe silver "growth" on copper bar.
Understanding: When the copper bar is placed in contact with the silver nitrate solution, there is a driving force to transfer electrons from the copper metal to the silver ions, oxidizing the copper metal to cupric ion and reducing the silver ion to silver metal. The silver ions are mobile in solution, while the copper atoms are immobile on the copper bar, so the reaction occurs at the surface of the electrode
Cu(s) + 2 Ag+(aq) → Cu2+(aq) + 2 Ag(s)
Expanding the view of the deposition on the bar, one sees a "fur" of silver precipitate that has formed. As the silver is deposited, at first the reaction occurs near the copper surface. As the reaction proceeds, the reaction interface becomes the surface of the deposited silver which grows outward in solution, creating the furry silver precipitate and a blue solution of cupric ion.Clearly, there is a driving force to reduce the silver metal and oxidize the copper atoms. Let's see how we can quantify the magnitude of the driving force by defining the reduction potential of each half-reaction.
Defining the half-reaction reduction potential
The reference electrode against which all other half-reactions are measured is the standard hydrogen electrode.
Each electrochemical half-reaction has a corresponding standard reduction potential which is a measure of the tendency for that element to be reduced relative to the standard reference half-reaction
2 H+(aq) + 2 e- → H2(g)
2 H+(aq) + 2 e- → H2(g)
EoH+|H2=0.0V
H2(g) + Cu2+(aq) → Cu(s) + 2 H+(aq)
EoH2|H+||Cu2+|Cu=0.34V
We say that relative to the standard hydrogen electrode, the reduction potential of the standard copper half-cell is 0.34V or
Cu2+(aq) + 2 e- → Cu(s)
EoCu2+|Cu=0.34V
H2(g) + 2 Ag+(aq) → 2 Ag(s) + 2 H+(aq)
EoH2|H+||Ag+|Ag=0.80V
Ag+(aq) + e- → Ag(s)
EoAg+|Ag=0.80V
We now have a burning question to answer. What is the voltage that would be measured for the electrochemical cell composed of a standard silver electrode in contact with a standard copper electrode? If we imagine that the electrochemical reaction is carried out reversibly, the work that is done in moving electrons from one half cell to the other is the work that is done in moving electrons across the voltage E
welec = -Q E
e x N0 = 1.602 x 10-19 C x 6.022 x 1023 mol-1 = 96,485 C/mol.
welec = -n F E
Now here is the kicker. We know that the change in Gibbs free energy for a chemical reaction at standard state will determine the position of equilibrium. The Gibbs free energy change is in general
ΔG = ΔH - Δ(TS)
ΔG = ΔH - TΔS
(constant T)
Δ H= ΔE + Δ (PV)
Δ H= ΔE + Δ (PV) = q - PΔV + PΔV + VΔP + welec = q + welec
(constant T and P)
ΔG = q - TΔS + welec
(constant T and P)
ΔS = qrev/T
ΔG = qrev - TΔS + welec = qrev - T(qrev/T) + welec = welec
(constant T and P for reversible process)
Δ G = welec = -n F E
(constant T and P for reversible process)
The relationship between cell voltage and Gibbs free energy
We have measured the standard reduction potentials of our silver half-cell and copper half-cells, relative to the standard hydrogen electrode. We now see that those reduction potentials correspond to changes in Gibbs free energy!
H2(g) + Cu2+(aq) → Cu(s) + 2 H+(aq)
Δ Go = -n F EoH2|H+||Cu2+|Cu
H2(g) + 2 Ag+(aq) → 2 Ag(s) + 2 H+(aq)
Δ Go = -n F EoH2|H+||Ag+|Ag
Cu(s) + 2 Ag+
→
Cu2+(aq) + Ag(s)
Δ Go =
-n F Eo
H2|H+||Ag+|Ag
- (-n F EoH2|H+||Cu2+|Cu) =
-n F EoCu|Cu2+||Ag+|Ag
Eocell =
EoAg+|Ag
- EoCu2+|Cu
Eocell =
Eocathode
- Eoanode
Δ Gocell =
Δ Gocathode
- Δ Goanode
EoCu|Cu2+||Ag+|Ag =
EoAg+|Ag
- EoCu2+|Cu
= 0.80V - 0.34V = 0.46V
Δ GoCu|Cu2+||Ag+|Ag = -n F EoCu|Cu2+||Ag+|Ag = -(2 mol) (96,485 C/mol) (0.46V) = -88,766 CV = -88.8 kJ
We can now see how the more cumbersome notation for the reduction and cell potentials pays off. The notation makes it clear that the standard reduction potentials are always for the reduction reactions. The addition and subtraction of half-cell reduction potentials, when labeled in this manner, will always lead to the correct result.
Ingredients: copper sulfate, zinc bar
Procedure: A complete recipe follows.
1. Prepare a solution of copper sulfate.
2. Place a zinc bar in the copper sulfate solution.
3. Allow a few minutes to pass and observe copper deposition on the zinc bar.
Understanding:
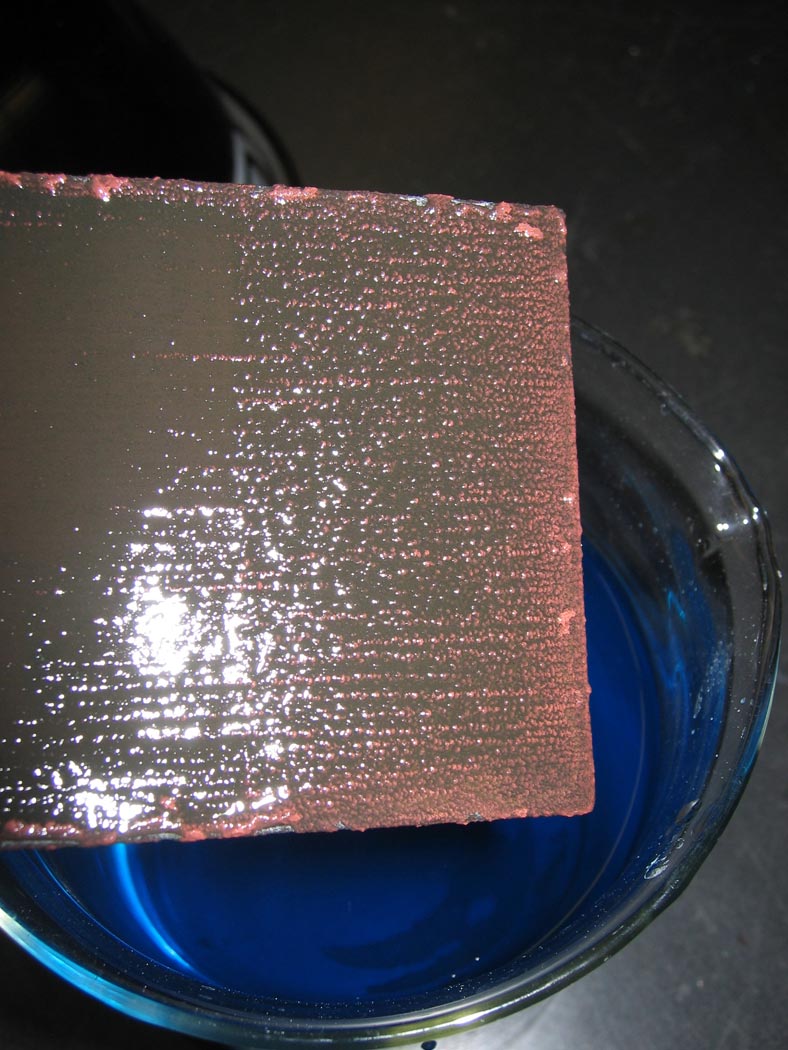
The spontaneous formation of copper metal deposited on the zinc electrode is rapid and dramatic.
The zinc metal is oxidized to divalent zinc ions, while divalent copper ions are reduced to copper metal.
The copper metal deposits on the zinc surface.
Zn(s) + Cu2+(aq)
→
Zn2+(aq) + Cu(s)
Δ Gocell =
Δ Gocathode
- Δ Goanode =
Δ GoCu2+|Cu
- Δ GoZn2+|Zn =
-n F EoZn|Zn2+||Cu2+|Cu
Eocell = 1.10V =
EoCu2+|Cu
- EoZn2+|Zn = 0.34V -EoZn2+|Zn
What does it mean that EoZn2+|Zn < EoCu2+|Cu? Since ΔG = -n F E we know that if E is positive then ΔG is negative and the reaction is favorable. So we can say that the more positive reduction potential of copper relative to zinc implies that copper will be reduced while the zinc will be oxidized.
In general we can organize the standard reduction potentials of a variety of metals
EoPt+|Pt > EoAu+|Au > EoAg+|Ag > EoCu2+|Cu > EoFe3+|Fe > EoZn2+|Zn > EoAl3+|Al
This result informs the use of zinc as a sacrificial electrode. Suppose that there is a metal such as iron that one would like to prevent from oxidative damage and corrosion. We can place the iron in electrical contact with a bar of zinc. As long as there is zinc metal, the electrons will always prefer to oxidize zinc while allowing the iron to remain in its reduced metallic state.
Galvanized steel is steel treated with zinc. The zinc is sacrificially oxidized, protecting the steel from oxidative damage. Anodized aluminum is aluminum metal that has been coated with aluminum oxide, subsequently forming aluminum hydrate. In that process, the aluminum surface is oxidized, preventing it from further corrosion.
You can check your answers here.
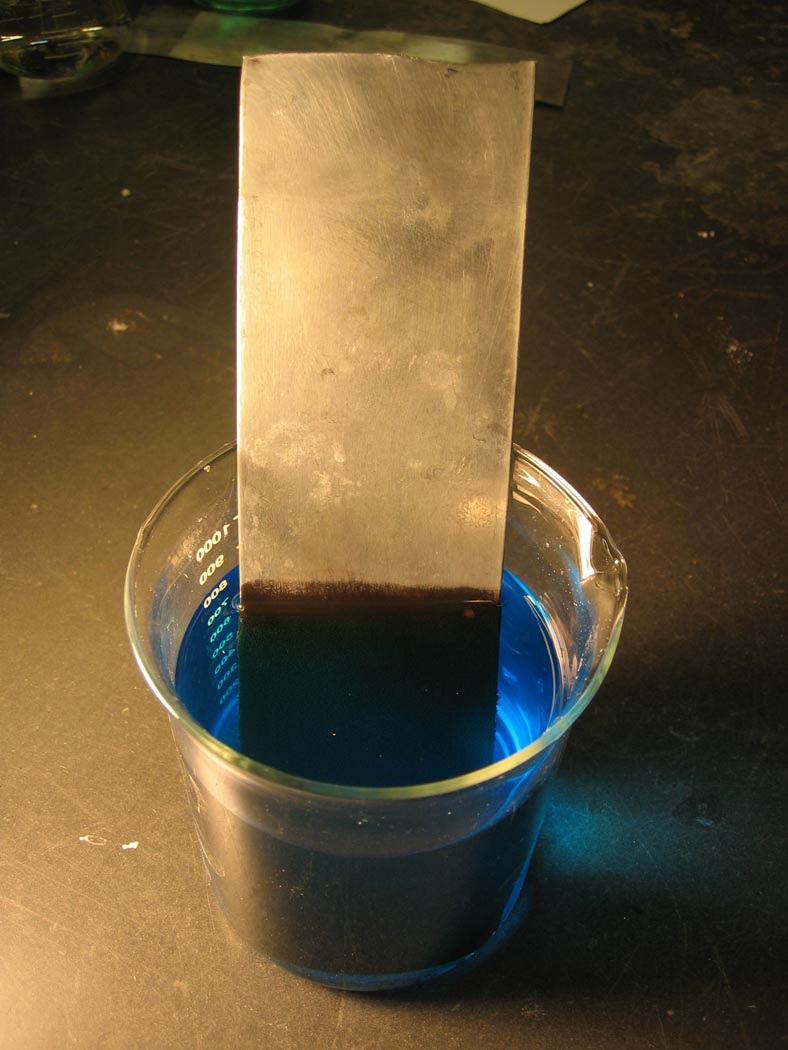 A zinc bar is placed in a solution of copper sulfate, leading to the deposition of copper metal.
A zinc bar is placed in a solution of copper sulfate, leading to the deposition of copper metal.
 What is the measure of the driving force of this reaction? We know that
What is the measure of the driving force of this reaction? We know that
Direct measures of relative reduction potentials
Question:
When the copper bar was placed in a solution of silver nitrate, silver metal precipitated out of solution, and the solution became blue with copper ion. What would happen if you placed a silver bar in a solution of copper sulfate?