Colorful formation of complex ions
A light pink solution turns blue, and then turns pinkish-blue ... lavender.
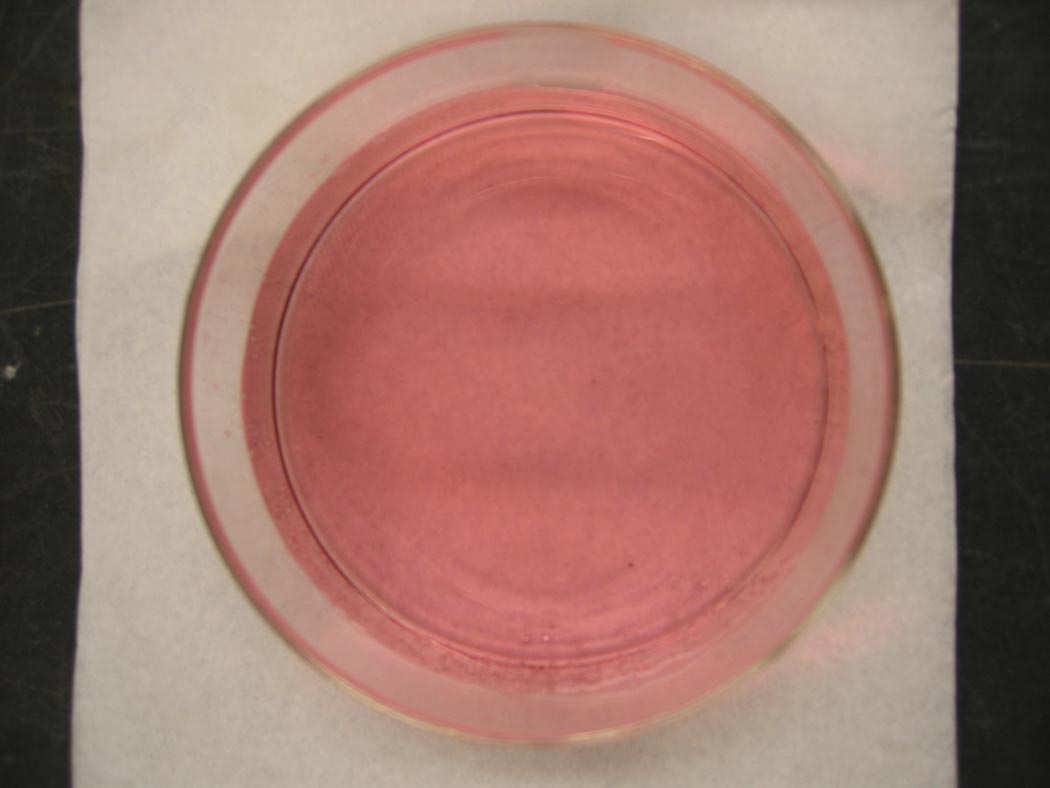
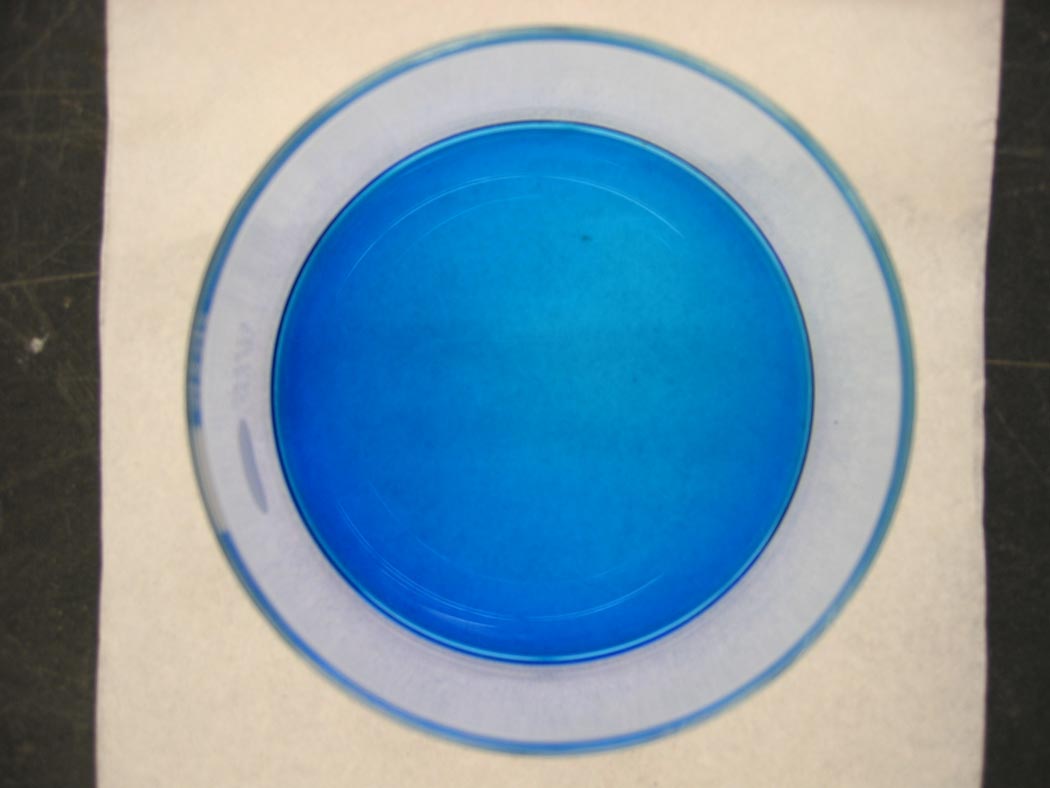
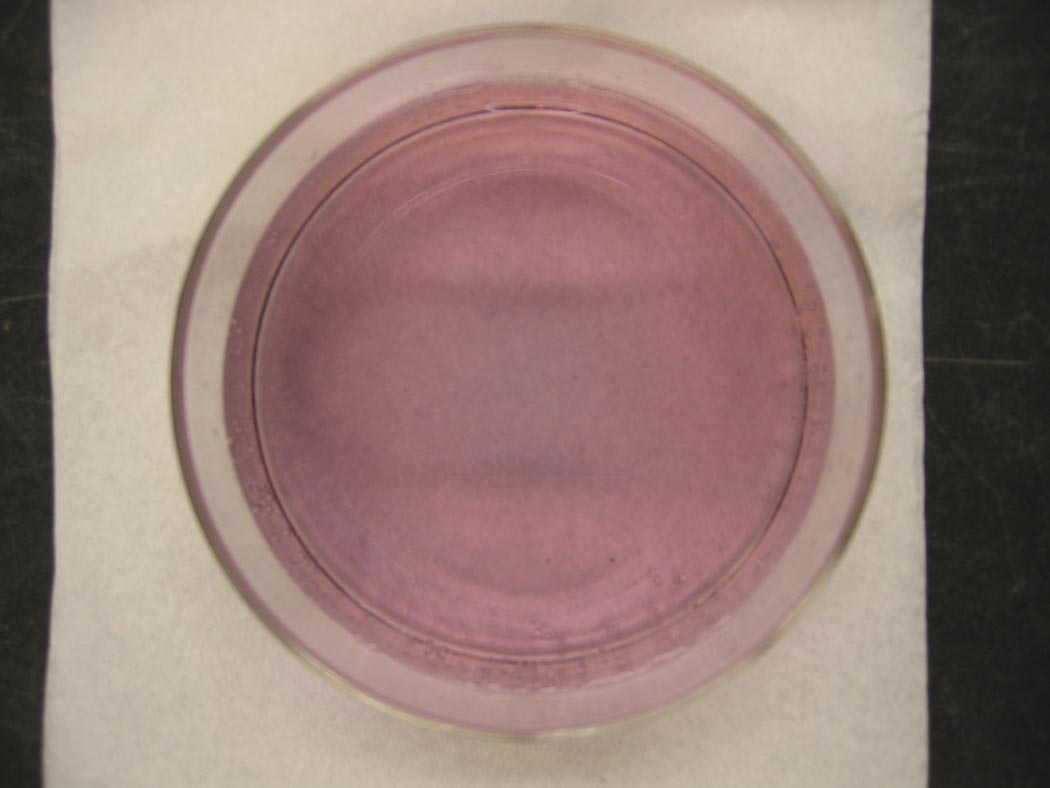
Ingredients: cobalt chloride, hydrochloric acid, water
Procedure: A partial recipe follows.
1. Prepare a solution of cobalt chloride in 98% ethyl alcohol.
2. Add hydrochloric acid to the solution and observe change in color.
3. Add water to the solution and observe transformation in color of solution.
Understanding: A solution of cobalt(II)chloride is a lovely pink color. The color is due to the presence of the pink hexaaqua cobalt(II) complex ion.
Co2+ + 6 H2O → [Co(H2O)6]2+
The addition of hydrochloric acid to the solution provides a high concentration of chloride ion. The addition of chloride ion drives the formation of the tetrachloro cobalt(II) complex ion.[Co(H2O)6]2+ + 4 Cl- → [CoCl4]2- + 6 H2O
Using our understanding of LeChatelier's Principle, we can push this equilibrium to the right, by the addition of more reactant, or the left, by the addition of more product.The addition of chloride ion pushes the equilibrium to the right favoring the formation of the blue tetrachloro cobalt species product. Addition of water to the solution pushes the equilibrium to the left favoring the pink hexaaqua cobalt species reactant.
If we add a sufficient amount of water to the blue solution dominated by the blue tetrachloro cobalt(II) complex ion, we can convert some of the tetrachloro cobalt(II) complex ion to hexaaqua cobalt(II) complex ion. The result is a solution with both pink and blue complex ions present, and a lavender color.
The quantum mechanics of complex ion formation
We have used quantum mechanical models to study the one electron atom, multielectron atoms, diatomic molecules, and polyatomic molecules. In each case, we have determined the discrete allowed energies of the system, and the one-electron wave functions or orbitals corresponding to each of the allowed energies. Using the Aufbau Principle, Pauli Principle, and Hund's Rule, we have were able to build the lowest energy ground state electron configurations for those systems. Through the electron configuration, we are able to develop a fundamental quantum mechanical understanding of the structure, ionization energy, bond strengths, magnetism, and spectroscopic properties of the atom or molecule. Quite powerful!
We would like to develop the same level of understanding of coordination compounds. We will find that a few simple rules provide us with a powerful means of understanding the detailed structure, magnetism, and spectroscopic properties of transition metal complexes.
The place to start is the central transition metal ion. We can determine the electron configuration of the ion alone. We can apply our rule of thumb that the ions of the first row transition metals have no 4s electrons. That leads to an electron configuration for Co2+ of
[Ar] 3d7
For the isolated ion, the five 3d-orbitals will have identical energies. However, when the ion is surrounded by the coordinating ligands, things change! In the case of the hexaaquacobalt(II) complex ion, the central cobalt ion is surrounded by six coordinating water molecules. Appealing to the rules of Valence Shell Electron Pair Repulsion Theory, we expect the water molecules to be arranged in an octahedral geometry. And they are!
The interaction of the water molecules with the cobalt ion can be understood using a localized electron model. In that model, a lone pair of electrons on the water molecule are donated to an atomic orbital on the cobalt ion. As such, the cobalt ion acts as a Lewis acid and the water molecule as a Lewis base. The cobalt ion forms six equivalent
sp3d2
The interaction between the coordinating ligands and the central metal ion can be understood in terms of two competing interactions. First, there is the attractive interaction of the charge and/or dipole moment of the coordinating ligand and the metal ion. That favorable interaction lowers the energies of the 3d orbitals. Second, there is the repulsive interaction of the donated electron pairs of the ligands and the electrons of the metal ion.
Complex ion formation and the spectrochemical series
Each surrounding ligand field will have a special geometry. In the case of the hexaaquacobalt(II) complex, the water molecules form an octahedral ligand field. The repulsive energy of interaction between the electrons in the water molecules will be different for different d-orbitals. For example, electrons in the atomic dz2 orbital will repel localized electrons on the coordinating waters positioned along the positive and negative z-axis of the octahedral ligand field. That repulsive interaction will raise the energy of the atomic dz2 orbital above that of the dxy, dyz, and dxz orbitals. The dx2-y2 orbital is similarly repelled by the octahedral ligand field. The repulsive interactions split the energies of the five 3d-orbitals on the cobalt ion into two groups, the lower energy dxy, dyz, and
dxz orbitals and the higher energy dx2-y2 and dz2 orbitals. The difference in energy between the two sets of d-orbitals is known as the ligand field splitting, &Deltao, for the octahedral complex
dx2-y2 dz2
Δo
dxy dxz dyz
The ordering of ligands from weak field to strong field is
Cl- < F- < H2O < NH3 < CN-
weak field strong field
Δo = hc/λ
We are almost there. We understand the shape of the complex ion, and the ordering of the 3d-orbitals on the cobalt ion. Now we need to build the electron configuration of the cobalt ion. We follow the Aufbau Principle, Pauli Principle and Hund's Rule and file six electrons first into the lower dxy, dyz, and dxz orbitals. We have one electron remaining and that can go into either of the higher lying dx2-y2
and dz2 orbitals, leading to an electron configuration
dxy2 dxz2 dyz2 dz21
dxy2 dxz2 dyz1 dx2-y21 dz21
Let's look at a few examples. If the cobalt(III) ion is surrounded by weak field chloride ligands, as in CoCl63-, we find that the ligand field splitting is small compared with the electron-electron repulsion. The electron configuration of cobalt will be found in a high spin arrangement.
If the cobalt(III) ion is surrounded by strong field ammonia ligands, as in Co(NH3)63+, we find that the ligand field splitting is large compared with the electron-electron repulsion. The electron configuration of cobalt will be found in a low spin arrangement.
What about the cobalt(II) ion surrounded by an octahedral ligand field of water? Water is an intermediate field ligand. The cobalt(II) ion is found to have a low spin arrangement.
You can check your answers here.
The quantum mechanics of a coordination complex
Question:
Consider the tetrachlorocobalt(II) complex ion. What is the electron configuration of the cobalt ion? What is the hybridization of the atomic orbitals on the central cobalt ion? Are the ligands that coordinate the central cobalt ion strong or weak field ligands? Is the cobalt in a high or low spin state? Is the compound paramagnetic or diamagnetic?