Colorful formation of complex ions
A light pink solution turns blue, and then turns pinkish-blue ... lavender.
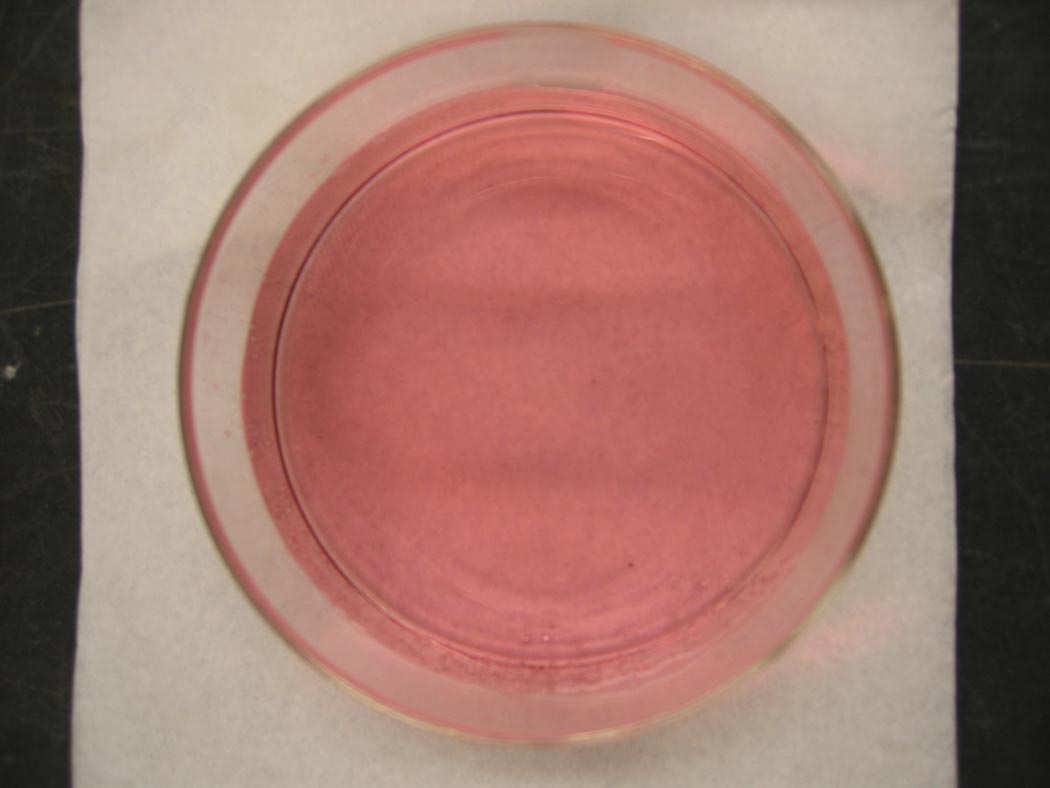
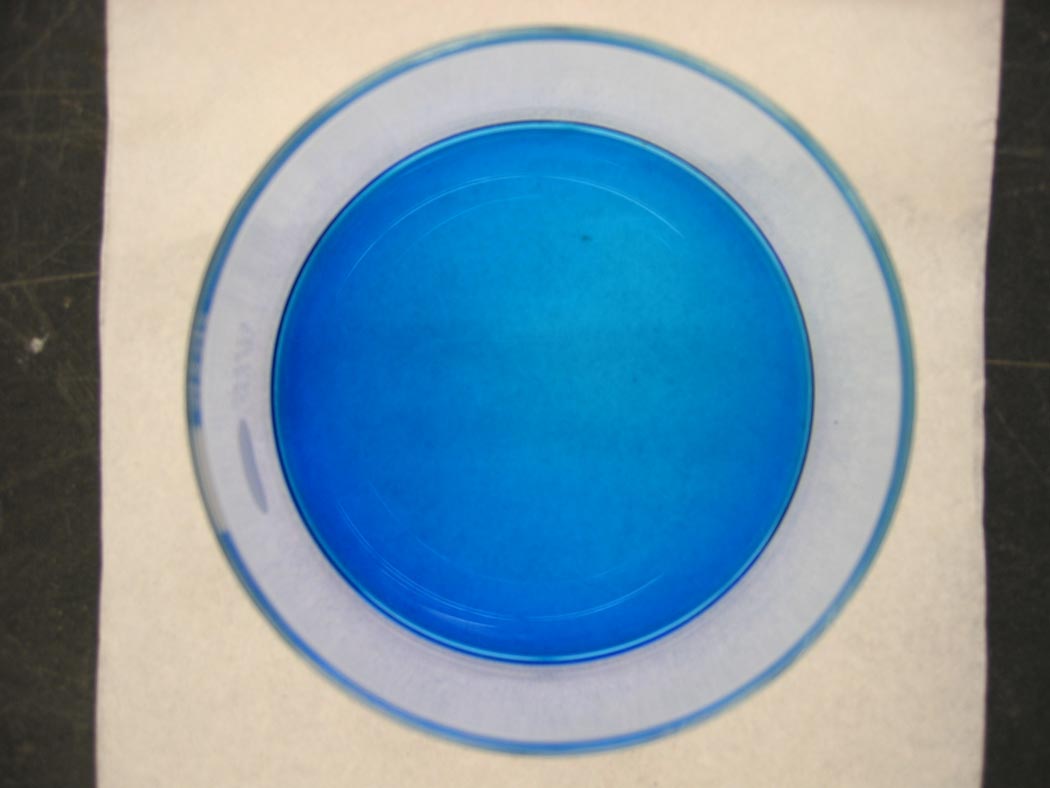
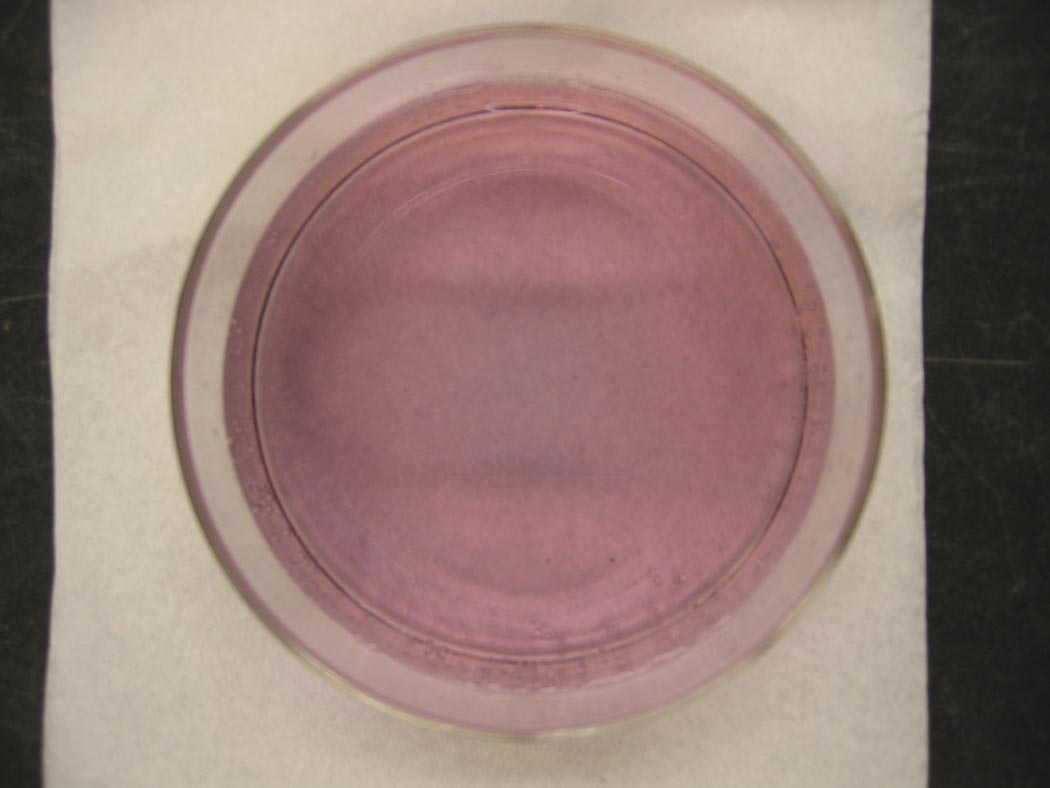
Ingredients: cobalt chloride, hydrochloric acid, water
Procedure: A partial recipe follows.
1. Prepare a solution of cobalt chloride in 98% ethyl alcohol.
2. Add hydrochloric acid to the solution and observe change in color.
3. Add water to the solution and observe transformation in color of solution.
Understanding: A solution of cobalt(II)chloride is a lovely pink color. The color is due to the presence of the pink hexaaqua cobalt(II) complex ion.
Co2+ + 6 H2O → [Co(H2O)6]2+
The addition of hydrochloric acid to the solution provides a high concentration of chloride ion. The addition of chloride ion drives the formation of the tetrachloro cobalt(II) complex ion.[Co(H2O)6]2+ + 4 Cl- → [CoCl4]2- + 6 H2O
Using our understanding of LeChatelier's Principle, we can push this equilibrium to the right, by the addition of more reactant, or the left, by the addition of more product.The addition of chloride ion pushes the equilibrium to the right favoring the formation of the blue tetrachloro cobalt species product. Addition of water to the solution pushes the equilibrium to the left favoring the pink hexaaqua cobalt species reactant.
If we add a sufficient amount of water to the blue solution dominated by the blue tetrachloro cobalt(II) complex ion, we can convert some of the tetrachloro cobalt(II) complex ion to hexaaqua cobalt(II) complex ion. The result is a solution with both pink and blue complex ions present, and a lavender color.
Lewis acids and bases
Question: The reactions describing the formation of the complex ions are acid/base reactions that can be understood using Lewis's theory of acids and bases. Identify the Lewis acid and Lewis base in each reaction.You can check your answers here.