Laboratory Safety Information
by
Jay A. Young
Chemical Safety and Health Consultant
12916 Allerton Lane
Silver Spring, Maryland 20904
Keynote address, 48th NEACT Summer Conference at the University of Main, Orono, Maine on August 18-22, 1986
Each of us has heard about some other teacher's serious accident to their student but unless it has happened to us, we are inclined to maybe pat ourselves on the back (while crossing our fingers) properly thankful that it didn't happen to one of our students and hoping that it never will. In these remarks, I'd like to make that hope a little more secure, a bit less uncertain. For those among you who have had a serious accident, I'd like to make it less likely that there will be another one.
So to begin. I'll ask for a show of hands: How many here have every had a close call? All of us have had at least one of these, including me. The main point I want to make is that a close call is a serious accident announcing in advance that it is coming, sooner or later -- unless you, and I, take corrective steps. Let's look at some statistics for accidents in general. These statistics are summarized in what is called the "Heinrick accident triangle." At the base of the triangle we have 100,000 hazards, or unsafe conditions. Statistically, these 100,000 unsafe conditions will cause 10,000 close calls. Saying it differently, if you had, say, three close calls last year in your lab teaching, you had thirty unsafe conditions. On the basis of 100,000 unsafe conditions, there will be 1000 recordable accidents, perhaps a burn from hot glass tubing or a spill that caused someone to slip and fall with a resulting broken arm, and so on. Turning this around, if you had three accidents last year that were serious enough to be reported, you also had 300 unsafe conditions that you did not know about and therefore could not correct, according to the statistics. The Heinrick triangle goes further. One accident that disables the victim signifies 1000 different unsafe conditions that have not been corrected. It is sobering to note that according to the statistics, a mere 30,000 unsafe unknown hazards will eventually cause one fatalist, if you or I have had one simple scratched student, which statistically signifies a mere close call, that incident strongly suggests also that there are ten hazards present that are not yet known to you or to me. But, one of those ten could be the cause of a fatality, not a mere scratch, next week or next month.
I agree that this is an extreme scenario: also emphasize that it its not implausible. The concept is straightforward. All accidents are caused. Every accident and every close call represents a large number of other causes that are unknown, unrecognized. Our task is to discover and eliminate every one of these unknowns to the extent that is possible. For this task, the principles of chemical safety will be helpful.
I. The Principles of Chemical Safety
The essence of chemical safety is comprised in four principles, each with a corollary and examples.
- Every chemical without exception is hazardous.
The corollary: "Hazardous" means possessing a potential to cause harm. The manner of use of a chemical determines the probability that harm is caused.
Examples: Oxygen inhaled at a concentration a bit or more greater than about 20% is poisonous. We take care to refrain from breathing oxygen at high concentrations for long periods of time. (Or, if you like, mother nature in this case has taken care of this for us.)
No one deliberately pours water at, say, 95°C on themselves.
- Every accident announces that it will happen before it happens.
The corollary: All accidents are predictable and therefore in principle preventable.
Examples: In the middle of the lab periods, a student says, "Ouch!" because they inadvertently but briefly toughed a piece of hot class tubing. That "Ouch" was the accident, a serious burn from hot class, announcing its forthcoming arrival.
You see a student briefly remove their safety classes in order to more conveniently read the meniscus level of a liquid in a graduated cylinder. And eye injury accident has just announced it is coming.
Spilled water, or other liquid, on the lab bench is not cleaned up within a reasonable times. Now you have been told that some one is going to slip and fall because of an un-mopped-up liquid spill on the floor.
- If it might happen, it will happen -- eventually.
The corollary: There is no such thing as personal immunity from harm. Each of us has said (me included) at one time or another, "I'll take a chance, it won't happen to me this one time." And, since we survived, it didn't happen; we beat the odds. Remember that when we say to ourselves, "I'll take a chance and do it because the probability of harm is very low," we are really saying that the probability of harm is not zero. Whenever the probability of an event is greater than zero, no matter how small, then it is certain that the event will occur. (It is foolish to hope that it will not occur to us.)
Example: When when was the last time you did not "buckle up" when driving your car?
- Each person is individually and personally responsible for the safe
use of chemicals.
The corollary: Use a chemical only after you have
a. Reviewed the kinds of hazards presented by that chemical.
b. Established the precautions that will minimize the probability of harm.
c. And, have prepared in advance for emergency measures in the event something goes wrong.
Example: Unless washed off by vigorous scrubbing under copiously flowing water initiated within 90 seconds after exposure, a spill of phenol on approximately 100 square inches of skin (e.g. a circle whose radius is a bit less than 6 inches) can be fatal.
II. Chemical Hazards, Precautionary Measures, Emergency Procedures
The hazards presented by any chemical depend upon the properties of that chemical. Since each different chemical is different from all others because it has properties that are different, it follow s that each chemical presents different hazards. To use a chemical properly, we must know the hazards of that chemical, the appropriate precautionary measures that reduce the the probability of harm from those hazards, and the necessary emergency measures (should our precautions fail) that also depend upon the hazards. Stated in these terms, the requirements are formidable. How can I know that much about each of the many chemicals my students will use in the lab -- to say nothing of teaching all this to the students themselves?
Fortunately, there is a practical answer. Chemicals present only four classes of chemical hazards and a fifth, physical, hazard in some instances. Although one chemical may indeed be more toxic say, than another, the precautions and emergency treatment depend principally upon the toxicity, not the degree of toxicity. The four chemical hazards are flammability, corrosivity, toxicity, and reactivity. IN what follows we will look at each separately and at physical hazards as will. Keep in mind that any single chemical may simultaneously present more than one of any of these five hazards.
A flammable chemical (obviously) will burn. Other terms that convey the same hazard potential information include"extremely flammable" and "combustible." Keep in mind that the vapors of flammables, if ignited when mixed with air in suitable proportions (ranging from 1%to more than 50% (by volume) in some cases) can explode. Flammable solids sublime hence their vapors are just as hazardous as the vapors from a flammable liquid. For example, glacial acetic acid (solid or liquid depending upon the temperature) is a flammable chemical as defined here. Keep in mind also that the vapors of most flammable are denser than air and can travel several tens of feet, or more mixing with air of course, but without dilution to less than 1%.
Precautionary measures include the enforced absence of ignition sources, such as lighted burners, heated surfaces (the class envelope of an ordinary lighted incandescent light bulb is hot enough to ignite some vapors), sources of sparks -- electrical including static charges and ignition sparks. Keep containers closed when not actually in use. Insure that the air movement in the laboratory is sufficient to keep the concentration of the flammable vapor in the air well below 1%. Minimize the quantities available -- usually 100mL is more than ample. Store in safety cans in an approved flammable liquid storage cabinet. Glass vessels (test tubes, flasks, etc.) that will contain flammable gases or vapors and handled by students or used by teachers in demonstrations should be taped beforehand to minimize flying glass shards, and used only behind a sturdy shield that will confine any flying parts.
Ask your local fire department to review the procurement, receiving, storing, handling, dispensing, use, and disposal of flammables and to make recommendations for improving safety.
Clearly, when using flammables, eye and face protection is mandatory, and, depending upon conditions, thick gloves and body and limb protective pads are also indicated. Make certain in advance that the safety shower is working and that students know how to use it. Insure beforehand that fully charged Class B fire extinguishers are available, that you and the students know how to operate, that there has been held a recent, successful, fire drill, and that the fire alarm system is operating and all persons know what the fire alarm bell sounds like. Depending upon the local conditions, you may wish to instruct students in the use of afire blanket, to "drop and roll" if their clothes catch fire, or to walk calmly to the safety shower, or some combination of these.
A corrosive chemical either destroys living tissue or causes permanent change in such tissue through chemical action. (A chemical that corrodes iron, for example, wet sodium chloride, is not corrosive under this definition -- which pertains to chemical safety. Ten molar sulfuric acid will corrode iron and is also a corrosive in a safety context.) Corrosives destroy skin and tissues under the skin. They destroy eyes, respiratory tract tissues, etc. The corrosive effects can be impaired sight or permanent blindness, sever disfigurement, permanent sever breathing difficulties, even death.
Usual precautionary measure include preventing contact with skin,m eyes, and respiratory tract. Wear eye protection and a face shield. The eye protection should fit around the eyes so as to prevent splashing around an edge and into the eye. The face shield should be a so-called full face shield, large enough, and curved, so as to protect the whole face, neck and ears.
Wear gloves made of a material known to be impervious to the corrosive being handled. Be sure the gloves are free of corrosive contaminant on the inside before wearing. If it is likely that bare arms will be splashed, wear sleeve gauntlets made for the same material as the gloves. Use a lab apron, made of a material known to be impervious and large enough and full-tailored to protect the clothing. An apron tied so as to not protect the lower neck/upper chest, or not long enough to protect the calf of the leg is inadequate.
III. Work Habits
- Never work alone in a science laboratory or storage area.
- Never eat, drink, smoke, chew gum or tobacco in a science laboratory or storage area. Do not store food or beverages in the laboratory environment.
- Never pipette by mouth.
- Wash hands before and after work in a science laboratory, and after spill cleanups.
- Restrain loose clothing (e.g. sleeves, full cut blouses, neckties, etc.) long hair and dangling jewelry.
- Tape all Dewar flasks.
- Never leave heat sources unattended (e.g. gas burners, hot plates, heating mantles, sand bathes, etc.)
- Do not store reagents and/or apparatus on lab bench, and keep lab shelves organized.
- Never place reactive chemicals (in bottles, beakers/flasks, wash bottles, etc.) near the edges of a lab bench.
- Use a fume hood when working with volatile substances.
- Never lean into the fume hood.
- Do not use the fume hood as a storage area.
- Obtain and read the Material Safety Data Sheets (MSDS) for each chemical before beginning any experiment.
- Analyze new lab procedures in advance to pinpoint hazardous areas.
- Analyze accidents for prevent repeat performances.
- Protection should be provided for not only the lab worker but also the lab partner working nearby.
- Do not mix chemicals in the sink drain.
- Always inform coworkers of plans to carry out hazardous work.
- Record who worked with what, when, and how long in order to allow meaningful retrospective contamination studies.
- Conduct regular in-house safety and health inspections with an emphasis on improvement rather than guilt
Safety Wear
- ANSI (or equivalent standard) approved eye or face protection should be worn continuously.
- Gloves should be worn which will resist penetration be the chemical being handled and which have been checked for pin holes, tears, or rips.
- Wear a laboratory coat or apron to protect skin and clothing from chemicals.
- Footwear should cover feet completely; no open-toed shoes.
|
O.E.H.&S.
Emergency Contact Numbers
"ACE" Plan
How to Use a Fire Extinguisher
Laboratory Safety Guidelines
Dress Policy
Electrical Safety
Minor Chemical Splash on Skin
Large Chemical Splash on Skin
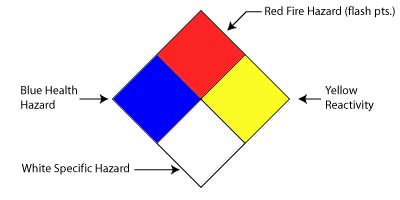
The 704 Diamond The 704 Diamond is required by the Boston Fire Department as a means to warn firefighters of hazards DURING FIRES in laboratories. Because these diamonds contain information that has been determined for FIRE conditions, these labels must be kept up to date. |
 |